- September 16, 2025
- By Chris Carroll
- Photos by John T. Consoli
A PAIR OF BIOLOGICAL realities helped shape Etienne Bilgo’s early life in the West African nation of Burkina Faso. He’d struggled to grasp one of them since first spending time with playmates: “Why was I alone when all my friends had brothers and sisters?”
He hadn’t yet reached high school when he decided scientific knowledge about reproduction might help him solve his parents’ fertility issues—painful in a culture that values large families. Though neither his mother nor father had progressed far in school, Bilgo soared to the top of his class, excelling in STEM courses.
This is where the second fact about his family’s biology might have given him an edge. While classmates often missed weeks of school with malaria, the mosquito-borne disease that sickens and kills more people per capita in Burkina Faso than almost anywhere else, he was spared.
Long after abandoning hopes for siblings, he learned why when he took a blood test before graduate school. Bilgo had inherited one copy of the sickle cell gene from his mother, a fortunate mutation that granted them immunity. (Had his father also carried the gene, however, it would have caused sickle cell disease.) The test cast his early years in a new, more fortunate light.

“I was surprised, and I thought about all my friends being sick from malaria; even my dad would get sick,” Bilgo says. “Thinking about this was what brought me to the field of malaria.”
He eventually became an expert in the topic of reproduction—of mosquitoes, not humans. Now a medical entomologist at a government scientific institute in Burkina Faso, he was the lead author of a study in Scientific Reports earlier this year including University of Maryland researchers that created what amounts to a sexually transmitted disease for the pest.
The research was one of the latest steps in an audacious, nearly 20-year effort led by UMD entomologist Raymond St. Leger to deploy a lifesaving new weapon against the disease, supported by the National Institutes of Health and other federal agencies.
The Distinguished University Professor has used genetic engineering to turn a common insect-killing fungus species into what he calls a “living insecticide” super-charged by spider genes that produce toxic venom. This cross-species addition allows the “transgenic” Metarhizium fungus to knock out malaria-bearing Anopheles mosquitoes so fast they can never spread the sickness. In their STD paper, Bilgo, St. Leger and other members of an international research team showed this could be done by infecting male mosquitoes with the fungus to pass along during mating.
The impetus for the UMD research is a continuing global rise in insecticide resistance among mosquitoes, along with the increasing resistance to antimalarial drugs in the parasite that causes the disease. Chemicals that have saved millions of lives in recent decades are teetering ever closer to failure.
“It’s an arms race between the mosquitoes and us,” St. Leger says. “Just as they keep adapting to what we create, we have to continuously develop new and creative ways to fight them.”
Etienne Bilgo, a medical entomologist in Burkina Faso who conducted research at UMD, records data on tests to control malaria-carrying mosquitoes. Bilgo has dedicated his career to fighting the insects that cause millions of illnesses and thousands of deaths in his country yearly. (Photo courtesy of Etienne Bilgo)

UMD entomologist Raymond St. Leger (center) works with then-student Brian Lovett Ph.D. ’19 to engineer a mosquito-killing fungus that they proved was effective in a paper the pair published in 2019.
IN THE RANKING of deadliest creatures that have ever preyed on humans, demographers agree that nothing—not sharks, saber-toothed tigers or even humankind with our growing array of weaponry—rivals the lowly mosquito.

While malaria is by far the most dangerous disease carried by some of the nearly 4,000 species, they also spread yellow fever, dengue, Zika, chikungunya and other serious
diseases, most of which are now increasing globally. The highest estimates indict mosquitoes for the deaths of half the humans ever born, perhaps 50 billion, mainly young children.
Proportionally, malaria fatalities today are far lower than historical highs, but 600,000 people still perish yearly of the disease. About 95% of deaths occur in Africa, as do most of the 250 million yearly infections; symptoms include fever, exhaustion and painful head- and body aches. The suffering is centered in heavily populated countries like Nigeria and the Democratic Republic of Congo, but little Burkina Faso, with only 23 million people, still tallies perhaps 10 million infections yearly and saw more than 16,000 deaths during 2023, according to the latest World Health Organization (WHO) statistics.
“The statistics are big numbers, but don’t explain what it is like having lived all our lives with this disease,” says Abdoulaye Diabaté, Bilgo’s boss and the head of medical entomology and parasitology at the Health Sciences Research Institute in Bobo-Dioulasso, Burkina Faso’s second-largest city. “There is a powerless feeling on the ground here that you can’t escape it. I don’t think many people outside of Africa can understand this burden.”
Diabaté is a global leader in malaria research, with scientific connections to major public health agencies, nonprofits and research universities around the world. As a collaborator in St. Leger’s research with the Metarhizium fungus, he coordinated his then-student Bilgo’s study at UMD in search of a fungal insecticide.
Source: World Health Organization
It’s an arms race between the mosquitoes and us.”
Raymond St. Leger
Distinguished University Professor of entomology
The institute is famous for developing insecticide-treated bed nets in the 1980s and ’90s; Diabaté assisted in some of the intense testing before the nets were distributed in growing numbers to the public earlier this century, when well over a million people died yearly from the disease. The bed nets stopped mosquitoes that fed on people while they slept, causing child death rates to plummet across Africa. But quickly, mosquitoes began showing resistance to the insecticides, and experts knew the reprieve would be temporary.
As public health efforts worldwide recalibrate amid recent funding cuts in the United States, long the global public health leader, experts say Africa may be a bellwether. The U.S. eliminated malaria more than 50 years ago with DDT and swamp drainage, but an invasive strain of malaria-carrying mosquito that’s well adapted to city life, Anopheles stephensia, is expected to spread as rising temperatures put more of North America within its range. Since 2023, authorities have tracked nine locally acquired malaria infections in the United States—the first in decades. A case of falciparum malaria (the deadliest form of the parasite) was detected in Maryland.
“The more prevalent malaria becomes here in Africa, the more likely it is to affect Europe and the United States,” Diabaté adds. “You can be sure it won’t just stay here.”

Burkina Faso’s MosquitoSphere, built by the country’s Health Sciences Research Institute with funding from the U.S. government, provides a safe outdoor testing site for St. Leger’s mosquito-killing transgenic fungus. (Photo courtesy of Etienne Bilgo)
THE WORLD DOESN’T WORK without fungi. During his postdoctoral work in the late 1980s, St. Leger began studying agricultural applications of species including Metarhizium, a type found worldwide that plays an important—and beneficial—role in the lives of plant species. With graduate student Gang Hu Ph.D. ’05, he showed that many plants would wither in its absence, unable to absorb enough nutrients from the soil or survive insect attacks.
(In recent research with former UMD postdoctoral researcher Weiguo Fang, now a regular collaborator based in China, St. Leger showed Metarhizium could effectively protect plants, along with the people and animals that eat them, from mercury poisoning caused by contaminated soil.)
St. Leger had been experimenting with genetically engineered versions that he suspected could be more effective and environmentally sound than chemical pesticides. But while serving on a Bill and Melinda Gates Foundation-backed committee in the early 2000s to look for technological approaches to aid African farmers, he had an epiphany. Instead of saving just crops, why not focus directly on protecting human populations?
“If I want to help people in Africa, I should help with this terrible burden of insect-borne diseases that is suppressing them—holding them back in innovation and weakening them economically,” he says.
Many forms of Metarhizium, rather than interacting with plant roots, infect and kill terrestrial and flying insects. St. Leger set out to modify the species to take on the specific mosquitoes responsible for spreading malaria and other tropical diseases.
I don’t think many people outside of Africa can understand this burden.”
Abdoulaye Diabaté
Head of medical entomology and parasitology at Health Sciences Research Institute, Burkina Faso
With postdoctoral researcher Chengshu Wang (now another regular research collaborator), he soon submitted the first paper about killing mosquitoes with a transgenic fungus in 2007 to Nature Biotechnology. First, however, he had to counter worries about dangers not to bugs, but to people. Foreshadowing the fictional plot of “The Last of Us,” a popular video-game-turned-HBO-hit-series about a fungal plague that wipes out society, reviewers suggested their innovation could comprise a “dual use” technology deployable as a weapon.
When finally published, the study showed that the addition of a neurotoxin from a scorpion increased the toxicity of the fungus to crop-eating caterpillars and a yellow fever-spreading Aedes mosquito by a factor of 22. But crucially, this surge in lethality left the “host range” unchanged, meaning the modified fungus remained dangerous only to the same creatures as the natural version—and no fungal zombies would be forthcoming.
In later experiments, St. Leger moved on to fungi that kill only mosquitoes; Bilgo had found the strain on insect carcasses in a small goat shed in Burkina Faso during his student days. Such precise targeting is one of the project’s layers of security; another is using toxins fatal solely to insects, while a third is ensuring that toxins activate only after the fungus has bored through the insect cuticle, injecting the poison deep inside.
An added benefit of using an existing “enemy” organism like fungi as an insecticide is that it should be more difficult for mosquitoes to become immune, versus establishing immunity to an inert, man-made product.
“The fungi have evolved their own strategies against the mosquitoes,” he says. Able to zig when the insects zag, “they’re an active player.”

Abba Gumel, a Nigerian-born UMD mathematician, created groundbreaking mathematical models to show how the fungus developed by St. Leger and colleagues could be used to control the disease.
WITH ITS MOSQUITO-KILLING bona fides established, it was time for the modified fungus to venture beyond a carefully controlled lab. Its inaugural “semi-field” trial played out in 2017-18 in a securely screened enclosure dubbed the MosquitoSphere, constructed by Diabaté’s institute in a small village near Bobo-Dioulasso with support from the U.S. National Institutes of Health.
Designed to simulate places where people encounter mosquitoes, the facility has puddles for them to breed in, small houses in which to buzz around (and potentially meet their fate via various insecticides) and domestic animals to feast on.
In 2019, Brian Lovett Ph.D. ’19, St. Leger, Diabaté and Bilgo published a key paper demonstrating that Metarhizium, which the researchers were modifying with the venom from an Australian spider, could cause the collapse of a mosquito population, killing the 75% of insects that contacted the fungus in the first generation and almost eliminating the second generation.
The results were promising, but the fungus would need a 90% kill rate to be useful. “It has to be shown it works not just in the sphere, but in the towns and villages,” Diabaté says.
The holdup now isn’t the need for further development of the modified fungus itself, St. Leger says. “Short of using synthetic biology to design a totally new pathogen from scratch, those science issues are done.”
A remaining step before moving on to open trials in towns across Africa—one required by the WHO and Burkina Faso’s government—is to present detailed plans and procedures for safely destroying insect populations with the fungus, along with solid evidence-based scientific studies pointing to likely success.
A study by another UMD collaborator, Distinguished University Professor Abba Gumel in the Department of Mathematics, could do just that. Gumel is an expert on using mathematical modeling and analysis to study the transition dynamics and control of emerging and re-emerging infectious diseases of major public health importance such as malaria, tuberculosis and pandemics like COVID-19. Today, he finds himself in the odd position of fighting one pathogen by encouraging another.
“I had malaria many times when I was growing up, so it’s not hard for me to get interested in this research,” says Gumel, born in Nigeria, a country with the world’s highest burden of malaria.
In a forthcoming paper in Applied Mathematical Modelling that expands on the mosquito STD research released earlier this year, Gumel worked with his former graduate student, Binod Pant Ph.D. ’24, and postdoctoral fellows Arnaja Mitra and Salman Safdar to assess for the first time the utility of the transgenic fungus to combat Burkina Faso’s murderous mosquitoes. Their modeling study, co-authored with Bilgo, Diabate and St. Leger, shows how regular releases of male mosquitoes (which don’t bite) infected with the fungus can effectively suppress local wild mosquitoes, significantly reducing malaria among people.
I had malaria many times when I was growing up, so it’s not hard for me to get interested in the research.”
Abba Gumel
Distinguished University Professor of mathematics
One daunting finding from the mathematical modeling: To work, the transgenic fungus approach requires 10 infected male mosquitoes to be released for every wild female mosquito in an area every few days for months. That adds up to many millions of mosquitoes until the disease is locally eliminated—a difficult public relations challenge.
“Male mosquitoes don’t spread diseases, but people see you releasing mosquitoes frequently and it makes them very nervous,” Gumel says. “The fungus-based approach wouldn’t be cheap or easy but if it reduces malaria burden, thereby saving lives in endemic areas, it would be well worth it.”
To achieve the 90% reduction in malaria cases and mortality, St. Leger and his collaborators aim to attack mosquitoes on several fronts with the transgenic Metarhizium. Targeting them through their reproductive behavior—with mating taking place outdoors—has the added benefit of slaying mosquitoes that have learned to seek prey outside to avoid pesticides in bed nets. Other new methods of applying the fungus would be less costly than mosquito releases; for example, a form engineered not just to kill, but to attract mosquitoes with floral odors, could be grown by residents at almost no cost and placed in traps or in the eaves of a house, St. Leger says. Yet another study has shown the transgenic fungus boosts the waning potency of chemical insecticides, indicating the new fungi can reinforce old approaches.
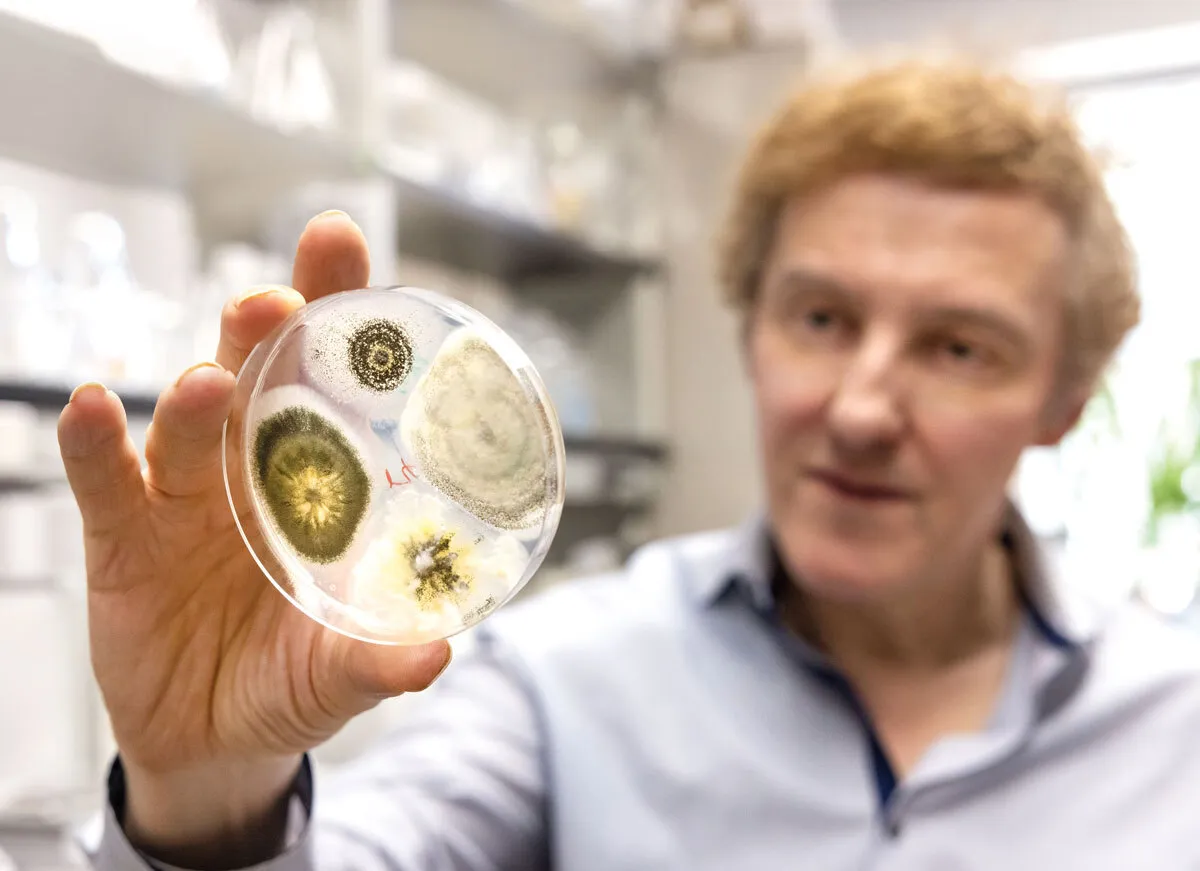
Thanks to mutations, four colonies of the same Metarhizium fungus take on different appearances in a petri dish that St. Leger holds in his lab. St. Leger boosted the abilities of the genus with genetic engineering, creating a “natural insecticide” he and colleagues hope will save lives in Africa and elsewhere.
FEW PEOPLE FROM AFRICA’S malaria-plagued regions can fully elude the disease’s ever-present danger—even those who are immune.
As Diabaté’s Ph.D. student, Bilgo studied for several months each year in St. Leger’s lab. This was in part because of the British American UMD scientist knew that for his potentially lifesaving, but controversial innovation to be accepted by those who stand to benefit, it would need introducing by respected African scientists.
As Bilgo prepared to leave Burkina Faso for College Park in 2014, he suggested to his steady girlfriend, a fellow graduate student, that they talk to their parents about an official engagement when he returned in a few months. She agreed. Around this time, she received what was likely an unnoticed mosquito bite.
When she died just days after his arrival in the U.S., Bilgo had no money to return home to be with friends or family. St. Leger remembers him enduring the blow with stoicism, then redoubling his efforts in the lab.
“Think about the mothers of the thousands of children in Burkina dying every year—I didn’t lose a child, so I didn’t have a right to feel sorry for myself. The death of a person I cared for was not why I went into malaria research, but all these deaths together ...” Bilgo says in a recent interview from Burkina Faso, trailing off.
Today, along with overseeing tests in the MosquitoSphere, he hosts local visitors as part of the plan to familiarize the engineered fungus to a population assumed to have deep doubts about genetically modified organisms.
But when the townsfolk see the hated insects falling dead at their feet, their skepticism about St. Leger’s fungus seem to drop as well.
“People are asking me, ‘Let me take this to my house,’” he says. “I have to tell them not yet, but I hope soon.”
UMD Research Changes Lives
At the University of Maryland, scientists and scholars come together to spark new ideas, pursue important discoveries and tackle humanity's grand challenges—improving lives in our communities and across the globe. See more examples of how UMD research changes lives at today.umd.edu/topic/research-impact.
This article is featured in the latest issue of Terp magazine. Read more at terp.umd.edu.
