- October 04, 2021
- By Kimbra Cutlip
A few years ago, scientists discovered that animal cell cytoskeletons—shapeshifting meshes of internal filaments that lend cells structure and help interact with their environments—would occasionally rearrange rapidly, setting off an earthquake-like disturbance in part of the cell. They dubbed the phenomena cytoquakes, but no one understood how or why they happened.
New computer simulations developed by University of Maryland researchers reveal that cytoquakes are caused by the slow buildup and sudden release of mechanical energy within cells, and may help them respond rapidly to signals from the outside environment, like chemicals produced by other cells or hormones in the bloodstream, they report today in the Proceedings of the National Academy of Sciences.
“We think these cytoquakes must be biologically important because the cytoskeleton is involved in so many functions within the cell,” said study co-author Garegin Papoian, the Monroe Martin Professor of Chemistry and Biochemistry with a joint appointment in the Institute for Physical Science and Technology. “Understanding the physics behind them can provide insight into how cells work.”
The cytoskeleton is like internal scaffolding, made of a network of filaments that constantly grow, shrink, attach and detach from one another. In addition to providing structure, the filaments also serve as tracks for chemical signals to flow from one part of a cell to another.
Papoian and his colleagues hypothesized that the sudden restructuring that happens in cytoquakes was the result of the cytoskeleton’s physical structure being particularly sensitive to its environment.
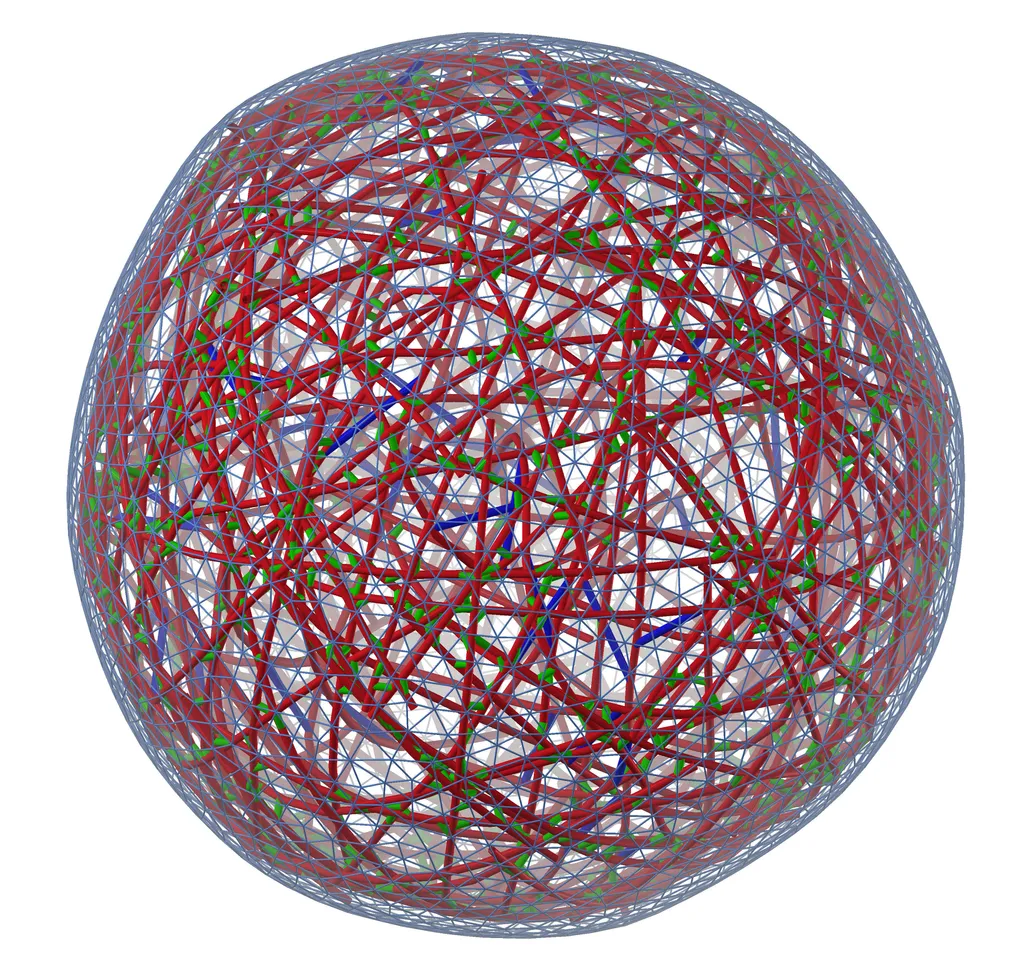
To test the hypothesis, the team created a simulation of a model cytoskeleton using a pioneering active-matter simulation software that they developed called MEDYAN, short for “mechanochemical dynamics of active networks.” The software applies the laws of chemistry and physics to predict how the molecules within the cytoskeleton interact and behave.
The study revealed that the filaments in a cytoskeleton arrange themselves a bit like a shape-shifting tensegrity structure—a kind of geometric toy or sculpture made of cables and floating rods under tension and compression that appears to defy gravity. Analyzing these structures helped Papoian and his colleagues understand tension release within the cytoskeleton. They found that tension applied to one area of the structure can build and cause tension until it suddenly releases in another area.
The physical structure of the cytoskeleton allows tension to build between some of the filaments like the tension between grains of sand in a sand pile or between two tectonic plates along a fault line in the earth’s crust. When some threshold is met and the tension suddenly releases, the pile of sand collapses, an earthquake rumbles or a cytoquake occurs.
But why do cells expend energy on cytoquakes?
“We postulate that the cytoquake mechanism poises the cell to react quickly to external signals from its environment compared to a system without this mechanism,” Papoian said.
For example, if a cell involved in repairing injuries must rush to the site of a wound, the cytoquake mechanism may respond to chemical signals from the injury site and jolt the cell into motion. When a cell migrates through the body, the leading edge may also use this mechanism to project or collapse protrusions to help it probe its local neighborhood.
The team’s next step will be to expand on their simulation methods to include more parts of a cell, such as the nucleus. They recently simulated the outer membrane of a cell and analyzed how the cytoskeleton pushes against this membrane to form finger-like protrusions.
“This work is showing us that we can use MEDYAN to model important components of a cell,” Papoian said. “Ideally, we would like to keep going and essentially build the fundamental model of a whole cell at single molecule resolution.”
Additional co-authors of the study from UMD include Carlos Floyd Ph.D. ’21 and Distinguished University Professor of Chemistry and Biochemistry Christopher Jarzynski.
